Atoms and Elements
What are atoms?
What do they look like?
What are they made of?
What properties do they have?
What are elements?
How could you see the shape of something too small to see?
So what did they see? What did the shadow tell them?

Note the the majority of everything is in the center. That area is called the nucleus of the atom. But the atom takes up a lot more space due to a shell or cloud of something around it.

Those parts are:
Proton - positively charged particle that determines what the element is (its properties). It has a weight of 1 AU and is found in the nucleus.
Electron - negatively charged speck that weighs almost nothing and is found in a cloud flying around the nucleus.
Neutron - neutral particle that weighs 1 AU and is found in the nucleus. They separate the protons from each other.
Not all atoms are the same. Sometimes an electron or neutron might be missing or extra. When that happens, the atom behaves differently. Let's investigate:
Ion - a charged atom due to extra or missing electrons.
Isotope - a differently weighted atom due to extra or missing neutrons.
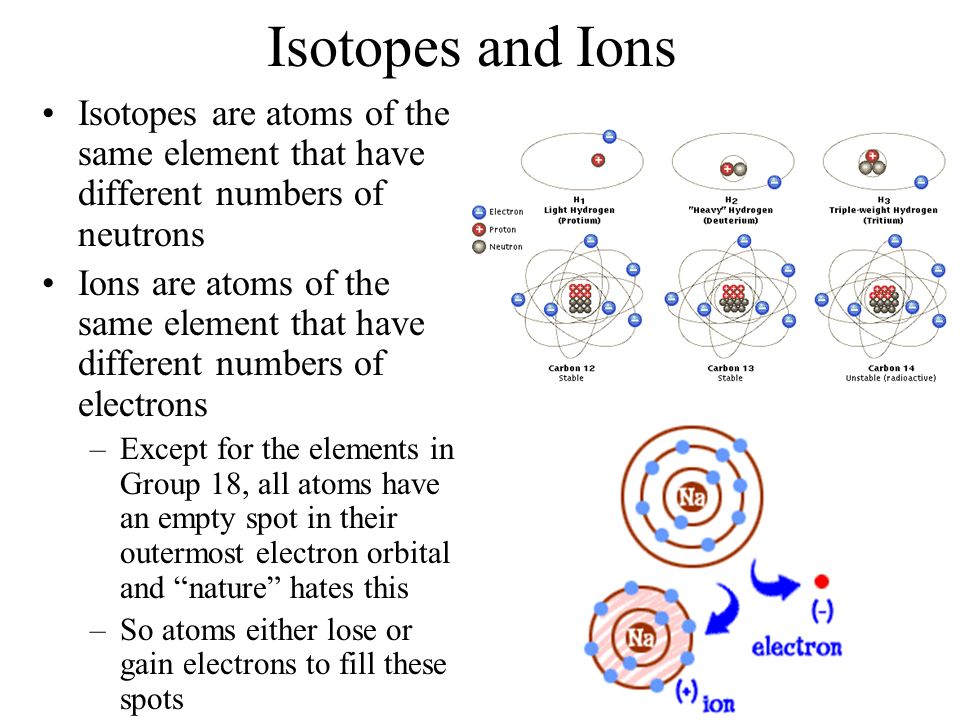
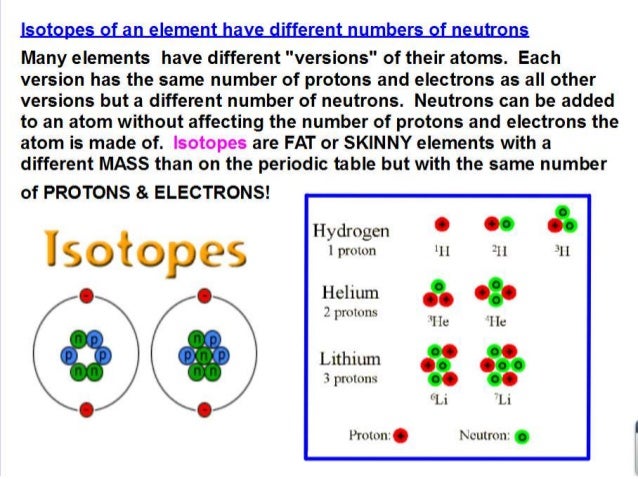


There are patterns of reactivity and stability based on the protons, neutrons, and electrons.
Let's investigate...
So the farthest column is stable (noble gasses). And the middle is relatively stable. But the edges are very reactive because the atoms have either one extra electron or one too few.
So as you go further down the periodic table, the atoms become less stable because the protons repel each other.
The middle sections are very flexible with regard to electrons and shape so they conduct electricity and are maleable.
Are you ready for the test?
- Draw and label an atom based on the periodic table of elements
- How does reactivity change across the periodic table of elements
- What is an ion?
- What is an isotope?