Chemistry
What is chemistry?
Do chemicals always react?
Why do chemicals react?
How can you tell when they do?
How can you speed up or slow down the reaction?
What are the different types of chemical bonds?
What is an acid? What is a base? What is pH?
The next unit we will explore is chemistry. This corresponds to pages 161-179 and pages 207-229 in the textbook. The atoms we have learned about are not always "happy" and often bind to become more stable. Stability comes with a full outer shell of electrons. Once they are happily bound, they have different properties. Chemistry is the branch of science that deals with what matter is composed of [atoms], their properties, and the ways they interact, combine, and change.
In other words: fun!
Give an example of an atom that is not chemically stable.
What about Sodium? 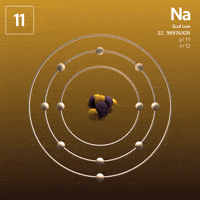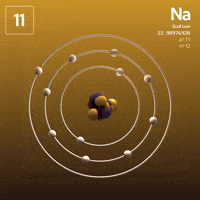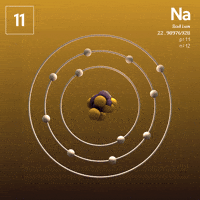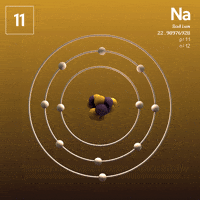 Sodium has that one lonely electron in its outer shell.
Sodium has that one lonely electron in its outer shell.
See that one electron all by itself flying around that outer shell, far away from the attractive nucleus? Wouldn't it be much more stable without it?
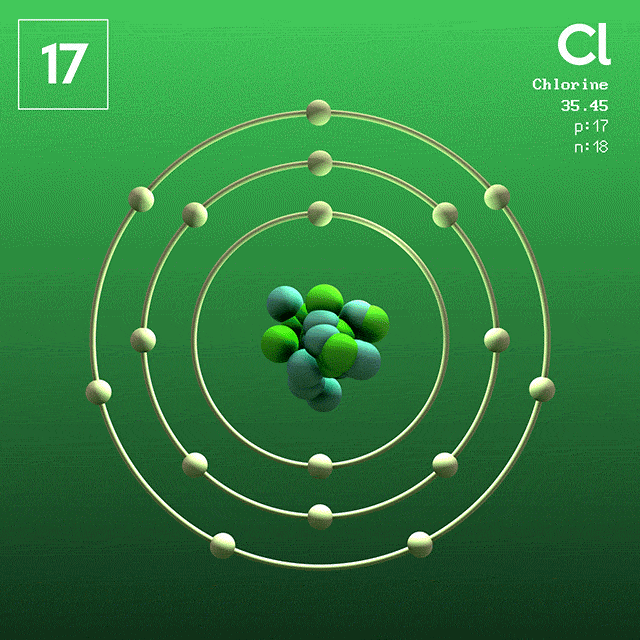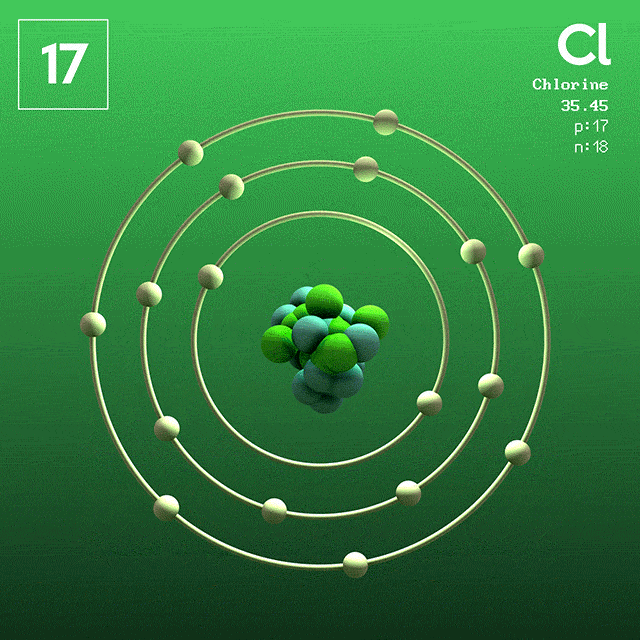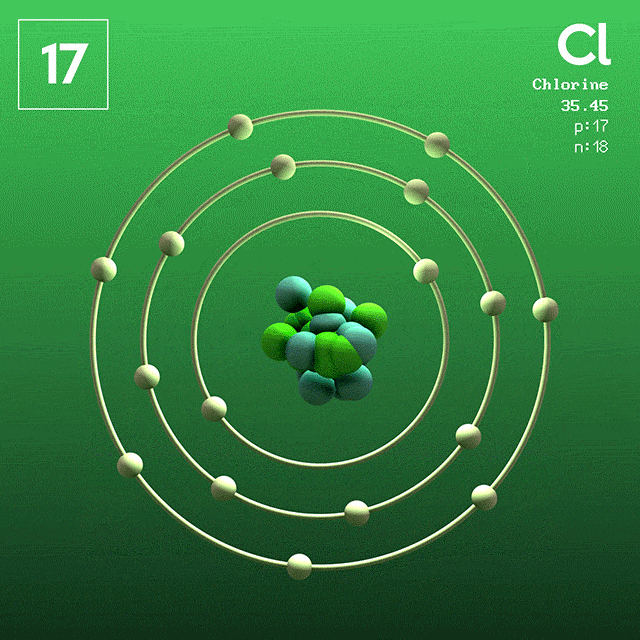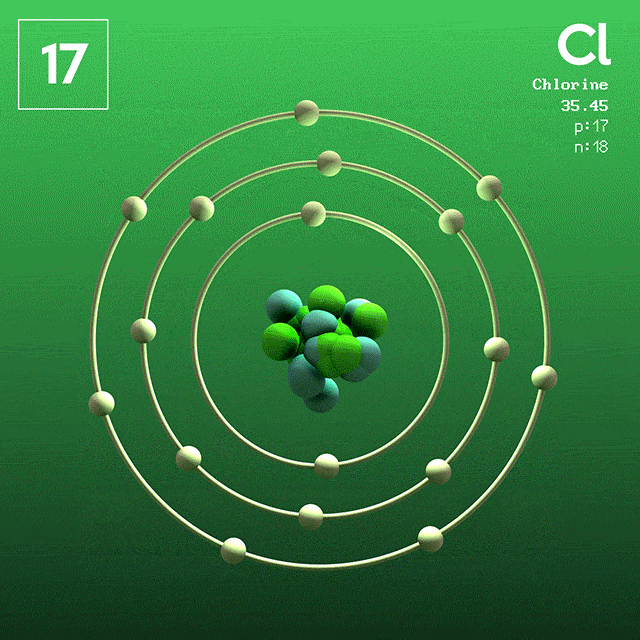
How about Chlorine?
Its outer shell has room for one more electron. With it, it would be happy and stable. So, when Sodium meets Chlorine, they react. The result:
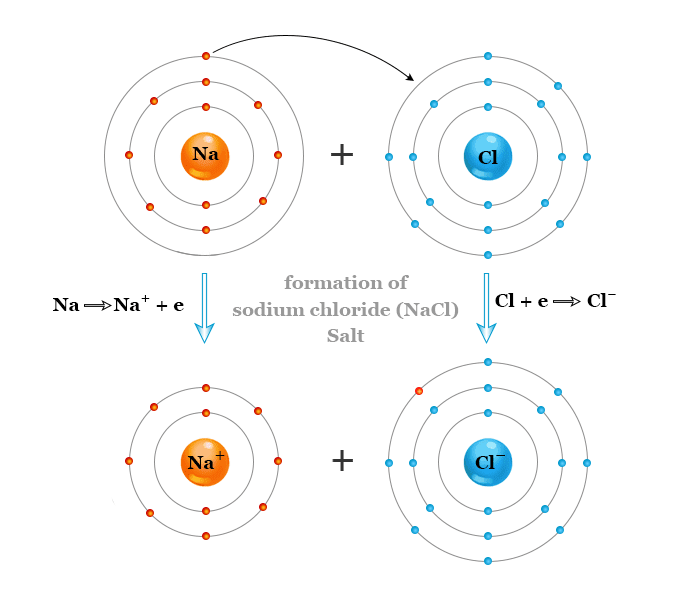
Now both are happy and they have had a chemical reaction.
This same bonding is behind lots of chemical reactions and is called an ionic bond. This is because both atoms become ions and are then attracted to each other.
Once they bind, the compound takes on an entirely different set of properties (i.e. Na & Cl --> salt or H & O --> water). You read that right: highly reactive and explosive metalic sodium combined with highly reactive toxic green gas chlorine combine to make table salt! Flammable hydrogen and flammable oxygen combine to make water that we use to put out fires!
There are different types of bonding. You just saw an ionic bond (where ions are made); there are also covalent bonds.
The formation of covalent bonds can be shown easily with electron dot diagrams. These simply show the outermost electrons of the atom as dots surrounding the symbol for that element. Another representation is a structural diagram. In these, a shared pair of electrons is represented as a line between the two atoms.
A closer look at the bonding:
So what is happening at the atomic level in these chemical reactions?
The atoms could be sharing electrons:

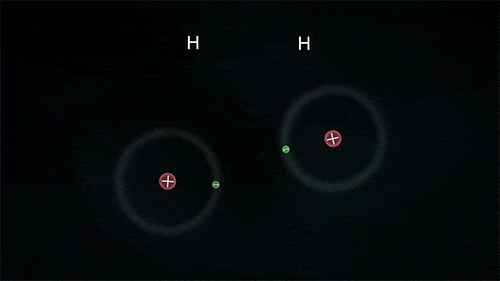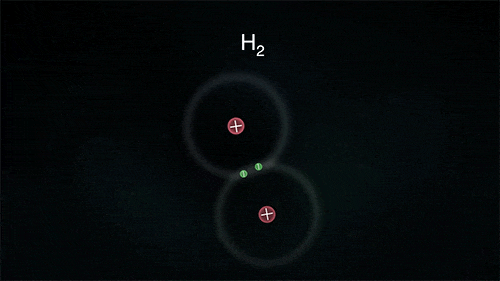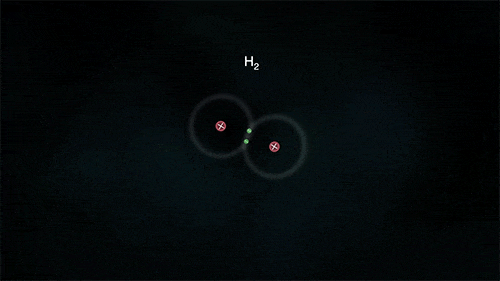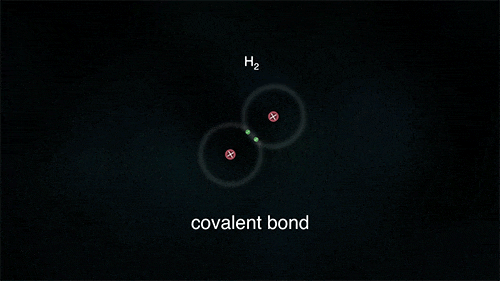
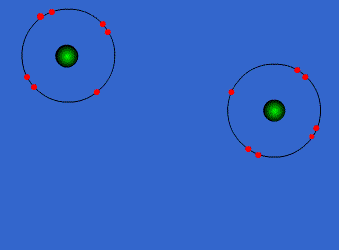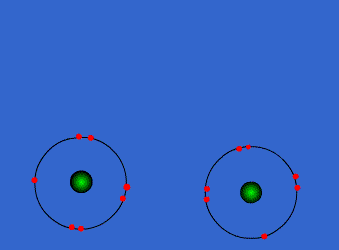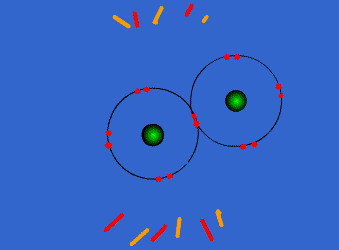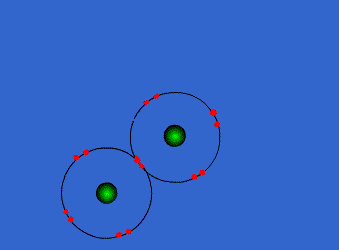 These atoms stay strongly connected because they share electrons
These atoms stay strongly connected because they share electrons
By sharing, both atoms have full outer shells and every atom is "happy" (stable).
This is why hydrogen and oxygen are reactive by themselves, but stable once bonded into a water compound.
An ionic bond is formed when one atom (usually an alkali metal) gives its electron to another atom (usually a halogen). This creates two ions that attract. This bond is looser than a covalent bond.
Some atoms do not share electrons, but instead give their electrons away and then are electrically attracted to the recipient:


 These give electrons away and then are weakly attracted to each other
These give electrons away and then are weakly attracted to each other
Once again, you start with extremely reactive sodium and chloride and end up with stable table salt.
The bonds an atom forms determines the shape of the object as a whole and therefore some of the new compound's new properties.
When two atoms bind chemically, they produce evidence of the reaction. There are ways to determine a chemical reaction occurred. We will explore these and look for signs.
Perhaps the first thing to identify is when a chemical reaction is taking place. Let's experiment and take good notes and see if we can tell when a reaction happens and when one doesn't occur.
Color change


Gas formation


Formation of a precipitate:
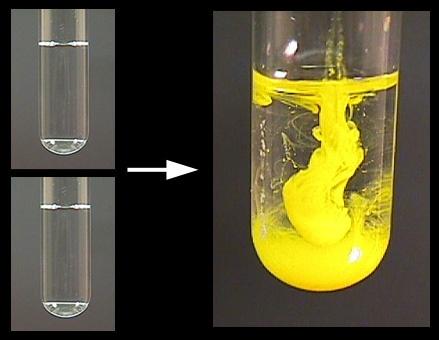


Change in temperature:


Giving off heat

Or absorbing heat

Other signs: smell, light, etc. 
Some of the mixtures do not react. These mixtures could be separated back out. Lets try to separate things:
A mixture is made of two or more substances combined in such a way that they can be easily separated. The atoms have not reacted with each other and therefore retain their own identifying properties. This is what happens when you mix salt and sugar. With time, you could separate them back out.
A solution is a mixture whose substances mix uniformly. The dissolving substance (like water) is called the solvent. The substance dissolved (like salt) is called the solute. The solubility of a solute is the amount that will dissolve in a given amount of solvent at a given temperature. Solubility increases as temperature increases.
Pour a little water in the bottom of a test tube. Tear a thin strip of paper towel. Draw a black line about an inch up from the bottom of the paper towel. Place the paper towel into the test tube with the mark side down (be careful not to let the black mark fall into the water). Set it aside and come back to it later.
Describe what happens to the black mark as the water moves up the paper towel.
How could you separate iron filings from salt?
How could you separate sand, salt, and water from each other?
A compound cannot be separated easily. It is made of two or more different elements that are combined chemically. This combination is always in the same proportion. The term molecule is used to describe any combination of atoms. Characteristic properties of compounds can be vastly different from those of the elements that combine to make it.

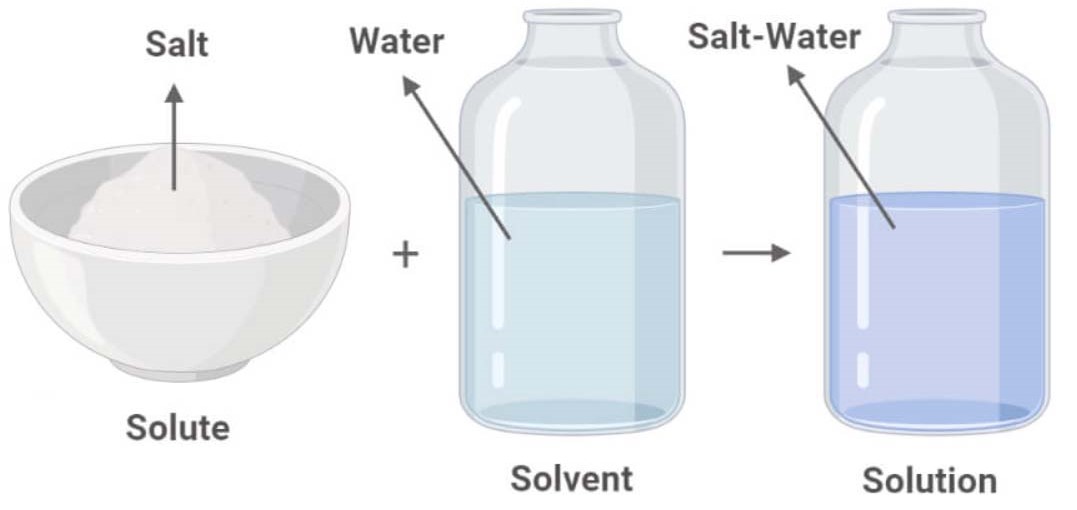
The SOLUTE dissolves into the SOLVENT to make a SOLUTION
Water is the universal SOLVENT because so many things dissolve in it
If a reaction is going to happen, it can happen only as quickly as the reactants combine. So, we can speed them up or slow them down.
There are at least four ways to speed up a chemical reaction.
How could you speed up a chemical reaction?
You could increase the speed of the molecules (i.e. heat it up)
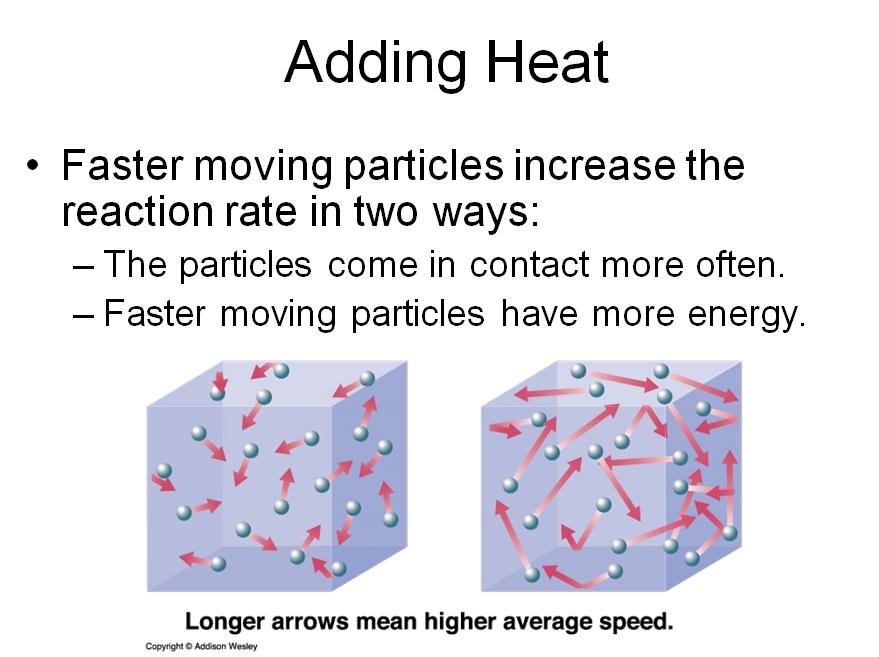
Since hotter things move faster and chemical reactions occur when the reactants come in contact with each other, increasing the rate that the particals move increases the reaction rate.
You could expose more of the reactants (i.e. increase surface area)
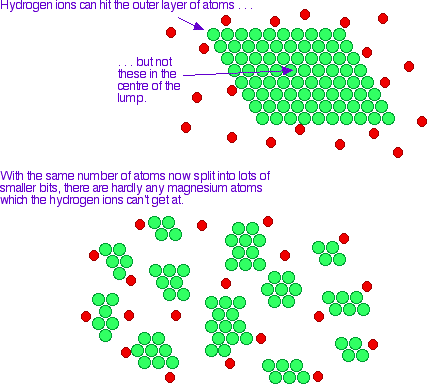
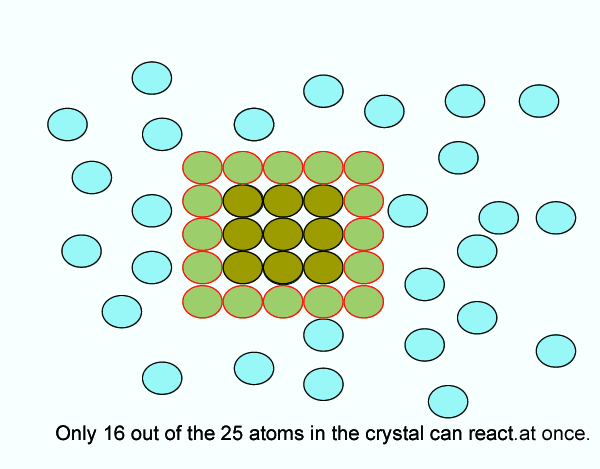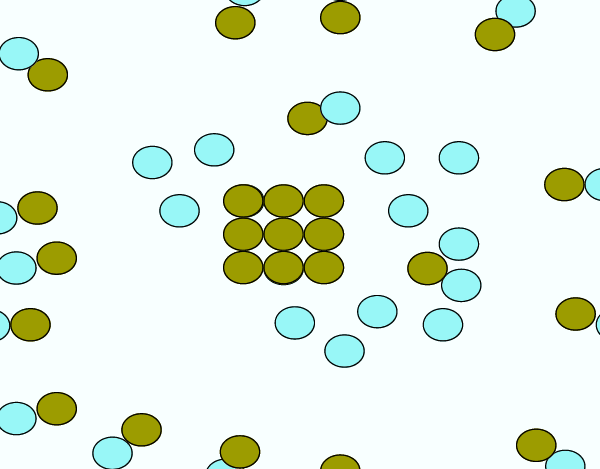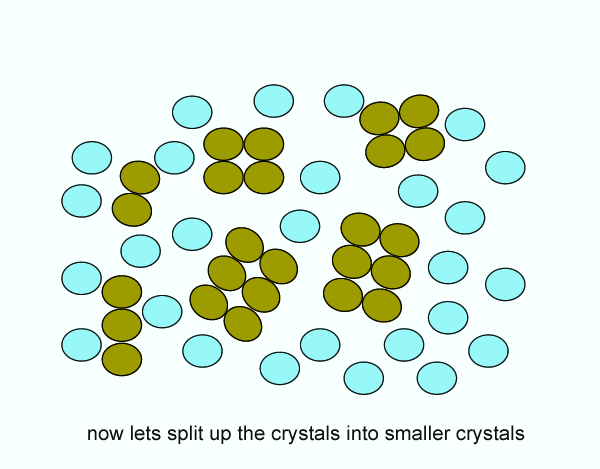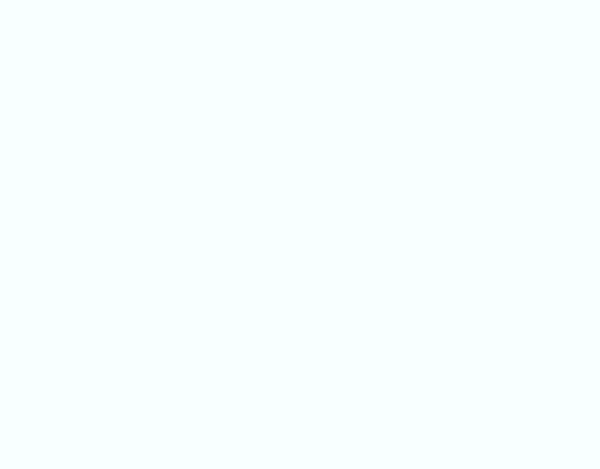
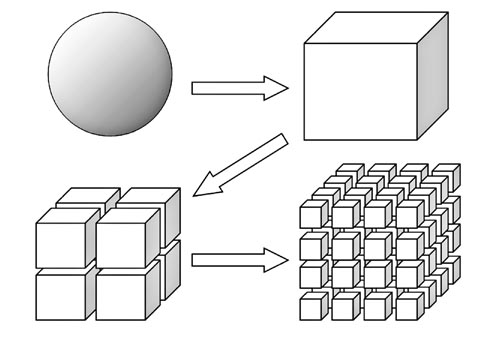
Since the reaction can only occur are the surface of the reactants, increasing the surface area yields a faster reaction rate
You could increase the number of reactants:
Having more reactants yields more reactions and therefore a faster reaction rate
You could add something that helps the reactants meet. These are called catalysts:
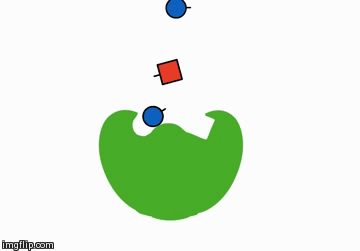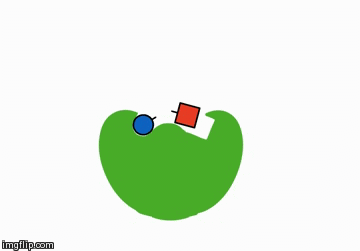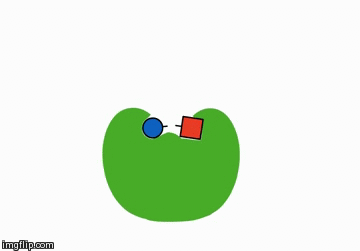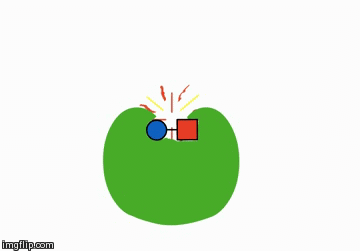
A catalyst is shaped in such a way that it brings the reactants next to each other and therefore speeds along the chemical reaction.
Actually, a catalyst is anything that speeds up a reaction without being consumed itself, but for the sake of this unit, the above explaination should suffice. We'll talk more about these when we learn about enzymes.
To summarize:
Special compounds like acids and bases need some further explanation. Definitions and examples:
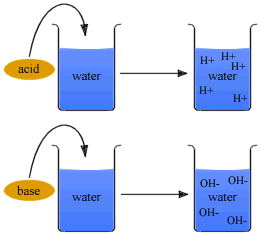
An acid is substance which produces positive hydrogen ions (H+) in water. A base is any substance which produces negative hydroxide ions (OH-) in water. The more concentrated the ion, the stronger the acid or base. Indicators are special compounds that change colors as the concentration of H+ or OH- changes. The strength of an acid or base is measured on the pH scale. The scale goes from 0 (most acidic) - 7 (neutral) - 14 (most basic) and is logarithmic. This means that a 2 is 10 times more acidic than a 3. Acids taste sour, bases taste bitter and feel slippery. Acids are usually a Hydrogen combined to a nonmetal (i.e. HCl). Bases are usually a Hydroxide combined to a metal (i.e. NaOH).
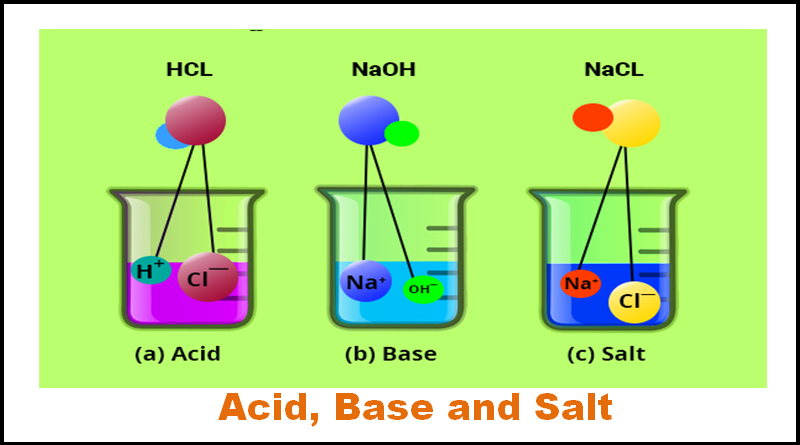
When you combine an acid and a base, they react to neutralize each other. This produces salt and water. This is actually the definition of a salt: the compound produced in a neutralization reaction when the hydrogen is replaced with another element from the base.
pH scale

Ionic bonds - Two ions of different charge attract each other (gives its e- to the other)
Covalent bond - Two atoms share the same e-
Compounds - different whole than parts (in proportion)
Mixture - two or more substances together, but not reacting with each other
Acids - release H in water
Bases - release OH in water
pH - scale of acidity: 1 - 14
Salts - ionic compound produced from acid + base
Organic - living
Are you ready for the test?
How would you know if a chemical reaction was happening?
Why do atoms react chemically?
Draw two atoms reacting.
How could you speed up a chemical reaction?
Contrast covalent and ionic bonds.
What is a mixture, solute, solvent, and compound?
Compare acids and bases.
What is pH and which pH is acidic?
If you have enjoyed the topics in this unit, feel free to investigate further. Here are some ideas. These are NOT required, but I hope you have fun and delve into some of them:
Continue exploring chemistry further. Dabble with a chemistry kit. Make something cool and bring it in to share.
Create an acid / base chart of common foods (did you know how acidic orange juice is?).
If hydrochloric acid is so strong, why doesn’t it eat away my skin when I spill it; what is molarity / molality?
Write another Dihydrogen Monoxide-like story. Be creative and have fun with it.
There is another type of bond called a hydrogen bond. What is it