Nuclear Energy
What is nuclear energy?
Are we going to learn about bombs?
What other (better) uses are there to nuclear decay?
The last unit in our exploration of matter and energy applies our understanding of matter’s atomic structure--nuclear reactions. This corresponds to pages 233 - 251 in the textbook.
This unit will look at the energy released by nuclear reactions.
Nuclear reactions are what happens inside the nucleus of atoms when they change into other atoms (gain or lose protons). Doing this allows them to be more stable (like filling an outer shell of electrons in chemistry). When they are more stable, they need less energy to exist and since energy does not just disappear, it gets released and we can use it. There are two types of nuclear reaction: fusion and fission.
First, we should review the atom:
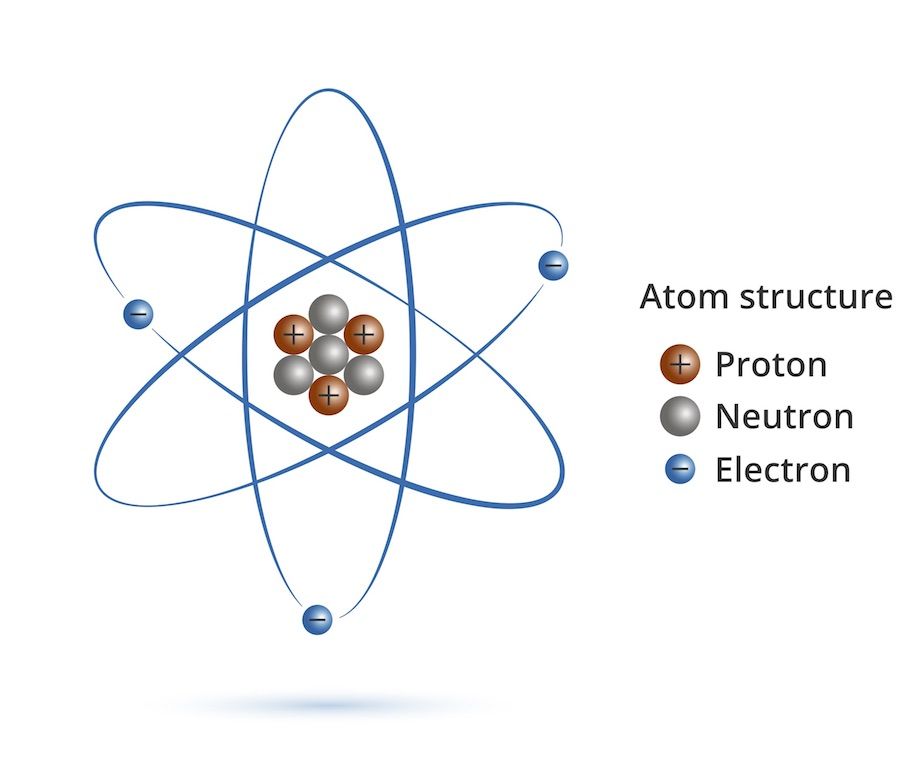
You will note that the Protons are all crammed in a tiny nucleus and have the same (repelling) charge. That means that they sometimes repel a proton out of the nucleus. This changes the atom and is called fission. Basically, if a proton comes flying out of an atom along with tons of energy, leaving a totally different atom than we started with (different number of protons), we call that fission. You see that the atom becomes more stable by not having as many repelling protons crammed in the tiny nucleus.
If you manage to cram two nuclei together, everything fits more compactly, thus needing less energy than having two separate atoms, and you get a totally new atom, we call that fusion. However you have to overcome the repelling shells of electrons so it takes lots of energy.

Let's look more closely at fission for a while:
Fission - 1 nucleus breaks down into 2 smaller nuclei while spitting out lots of energy. Is there anything else that comes out during this decay?
Fission will occur naturally with any atom that doesn’t have enough “peacekeeping” neutrons or may be accelerated by neutron bombardment. That means that if an atom is likely to decay on its own, if we hit it with a neutron, we can make it break down then.
How much energy is released? A fuel cell the size of this “O” has the equivalent energy of burning 1000 kg (over a ton) of coal!
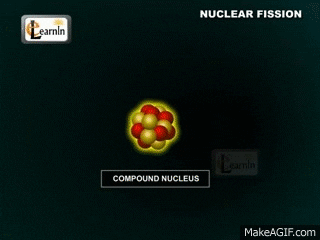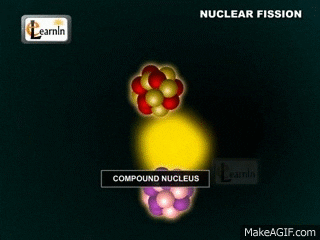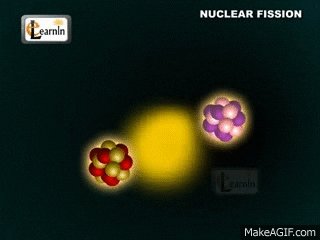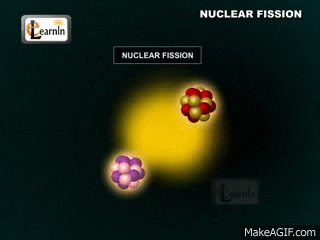

Note that two "daughter" atoms are produced. As are three neutrons. This breakdown happens at a set rate (the rates are different for different atoms, though). Sometimes Alpha particals are released (stable heliums). Sometimes beta particals are released (an electron (or positron). Gamma rays (energy in the form of waves) are always produced. These gamma rays are the form of energy we usually capture.
The daughter atoms are new (hopefully stable) atoms.
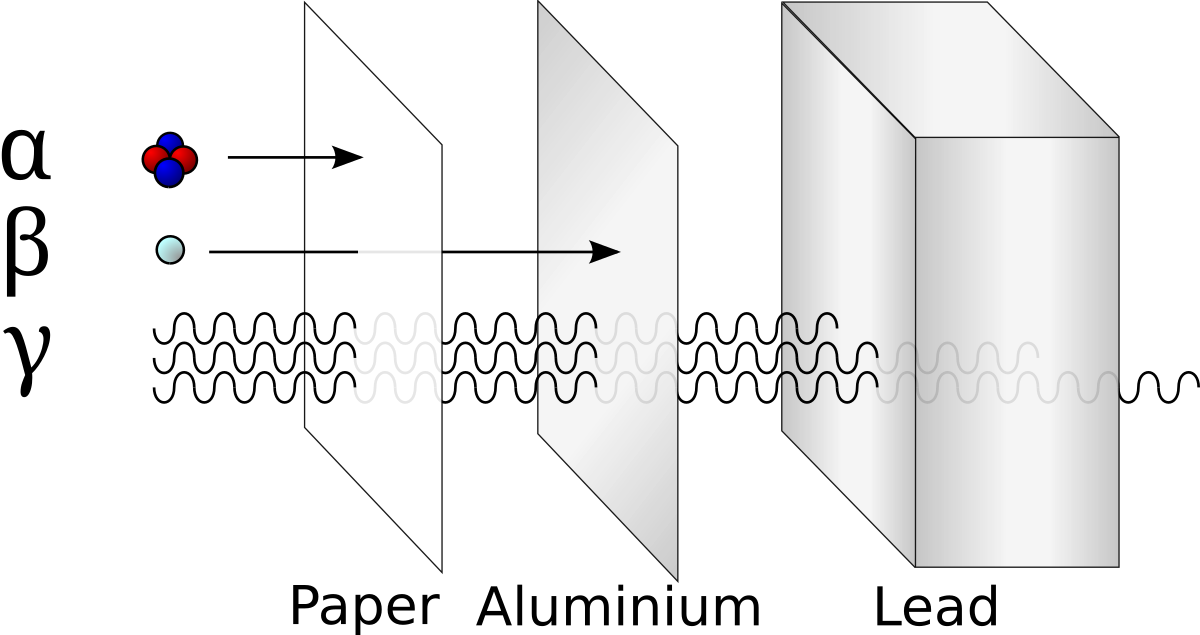
Alpha particles are big and have the most energy, but are easily blocked by cloth or paper.
Beta particles are smaller and less energetic. They are blocked by aluminum foil.
Gamma rays may be the least energetic, but are so small that they get through more things. They are only blocked by lead (like when you get an X-ray) or concrete (like in the walls). They also are released in all decay, so they are far more common.
This energy can break bonds.
If living this causes: fatigue, loss of appetite and hair, mutations, cancer, and death.
If not living, just weakens the structure.
So fission produces daughter atoms at a constant rate, three nuclei, and energy, plus sometimes other things like heliums and electrons.
These products can be used in many, many ways! Here are some ways humans have used these products:
A nuclear chain reaction progresses because the neutrons shot out by the decay bombard the other atoms and cause their decay. They can either be controlled or not.
Uncontrolled chain reaction - huge amount of energy in a very short time = BOMB!
Controlled chain reaction - many of the emitted neutrons are absorbed away to slow the decay down. This is what occurs inside a nuclear power plant. They have rods of U235 with control rods to absorb the extra neutrons. The gamma rays heat up some water which in turn turns a turbine (a magnet inside a coil of wires).
Bombs:

We discovered that hitting a fissile atom with a neutron makes it break down. And the breakdown releases three more neutrons. If we let each of these hit more atoms then we get a chain reaction releasing all that energy in a short time (bomb!).
Nuclear power:
This is the same as a bomb except with some rods that absorb some of the neutrons so that it does not get too hot.
Concerns with nuclear power are:
Accidents like Chernobyl and wastes that will be highly radioactive for the thousands of years.
Benefits of it are:
Cheaper to run and it does not release harmful gases into the atmosphere.
Medical tracers:
Since we can detect these energetic particals, if we put some in a person, we can see where they go.

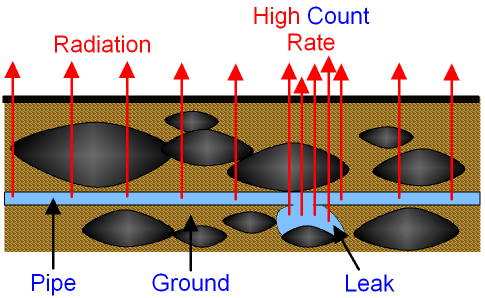
Detecting leaks:
If we put these detectable things in a tube, we can detect any leaks.
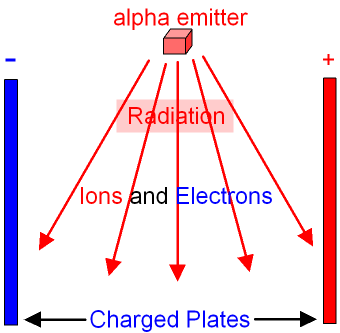
Smoke detectors:
If our detection of them gets blocked, that means that something large just got in the way. Since paper blocks alpha particles, we can detect smoke easily:
Cancer treatment:
Because radiation causes mutations and most mutations kill the mutated cell, if we blast cancer with radioactivity, we can kill the cancer.
So radiation is used to fight cancer.
Irradiation:
If we blast water with radiation, it mutates and kills any bacteria in that water, so we can purify water. This can be done for food as well to help us preserve food better.
Dating:
Because the breakdown is at a constant rate, we can date something by the ratio of parent atoms to daughter atoms:
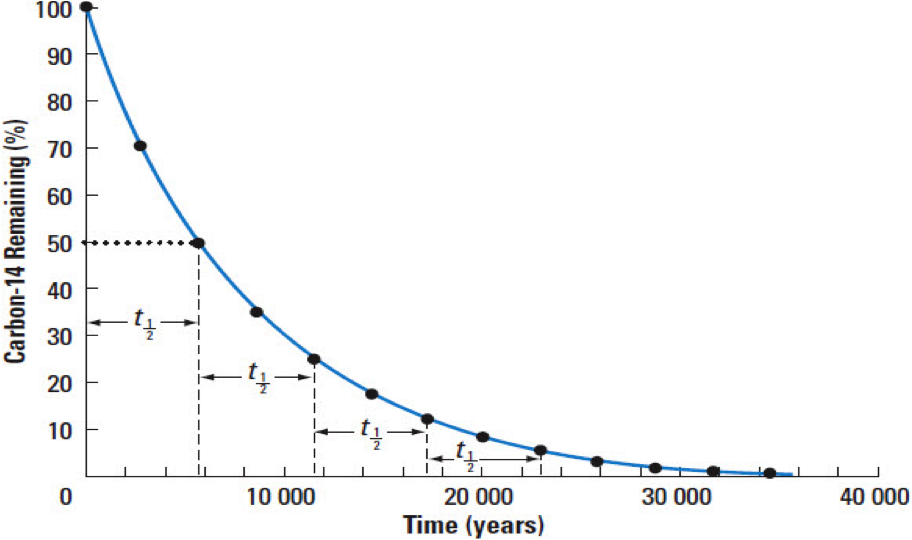
Radioactive substances decay (undergo fission) at a set rate called a half life. One half life is the time it takes one half of the substance to decay into daughter atoms and it is constant for a specific material.
Looking at the three neutrons that shoot out (chain reaction?), the alpha, beta, and gamma energy that is released, and the rate of parent / daughter atoms, what other uses can you find for radioactive decay?
Fusion:
2 or more nuclei combine to form one larger nuclei (they fuse into one)
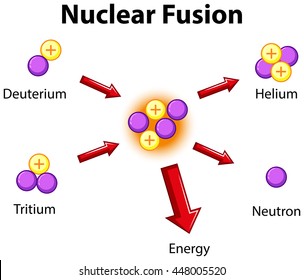
Did you notice what the fuel for fusion was?
hydrogen!
Did you notice the waste product for fusion?
helium!
Actually, you could use any fuel and produce any product. Burning helium to make gold, anyone?
So why are we not doing this?
What is stopping atoms from fusing?
The repulsion of the electrons and the tiny nature of the nucleus are the major hurdles in the way of fusion. If we heat something to plasma (ions with no electrons around and moving very quickly), or if we speed them up really, really fast, we can get them to fuse. Both require tremendous energy input by us. So much energy that it is not worth it. But if we could perform cold fusion...
The proton repulsion also must be overcome meaning that very high temperatures are required (around 100,000,000 Celsius). At this temperature, everything is plasma.
Advantages of fusion for energy:
There is a much smaller chance of an accident, the waste products are not harmful, there is tons of fuel for it, and more energy is released per mass.
Fusion is the most common energy source in the universe because it is happening in every star.
This is the large Hadron Collider at CERN:
It measures 17 miles long! In it, they can ram two atoms into one.
Are you ready for the test?
What are the two types of nuclear reactions?
Contrast fusion and fission.
How would you know if an atom was likely to undergo fission?
Name the three radioactive particles / energy that are emitted during nuclear reactions.
What is a half life?
What are the advantages and disadvantages of the two types of nuclear reactions for
energy?
What are some of the many uses for nuclear reactions?
Hypothesize how to create that “Holy Grail” of nuclear physics--cold fusion.
If you have enjoyed the topics in this unit, feel free to investigate further. Here are some ideas. These are NOT required, but I hope you have fun and delve into some of them:
How does a nuclear power plant really work? What happened at Chernobyl? Three Mile Island?
Find out more about half lives. Which ones do we use to date the age of the Earth? A dinosaur fossil? The first human artifacts?
Do you have any ideas on how to “clean up” the nuclear wastes?
Which is better: nuclear power that produces radioactive byproducts that take thousands of years to decay, or fossil fuels that produce carbon dioxide (causing global warming)? Why?