Matter
The first unit we will explore this year is matter. Since we will be learning physical science and physical means something we can touch, it makes sense to start with what makes up all these touchable things in the universe--matter.
This corresponds to pages 29 -35 & 100-105 & 108-111 in the textbook.
We will learn what matter is, the states of matter, and conversions between the states of matter. Then we will investigate some properties of matter (like density) and specific states of matter (like liquids). We will learn Archimedes principle, Bernoulli’s principle, how flight works, and a little about pressure. Hopefully we will answer: Why do some things float, others do not, and a submarine can do both? What keeps airplanes and hot air balloons up and what gets them down? What is with those suits astronauts wear? Why is a weather balloon only partially inflated before being sent into the upper atmosphere?
So let us start with defining what we will be studying:
What is matter? (remember from the last unit)
Matter is anything that has mass and takes up space. Mass means it is composed of stuff. You can think of it as the object's weight (although weight is slightly different--it is how much pull between an object and the planet). How much space something takes up is called its volume. It is how big the object is. So matter is simply anything that is big and weighs something. However "big" and weighs "something" can be extremely tiny and light. If we can measure its mass and size, it is matter.
There is a property of matter that we will come back to later that relates an objects mass to its volume. It is called "density."
Matter comes in different forms and has certain properties that can be fascinating.
What forms or states does matter come in? What are the states of matter?

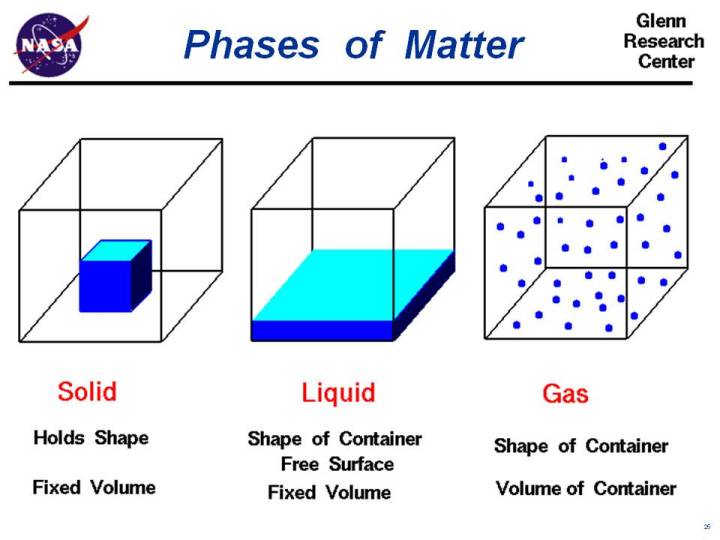
Solids are made of atoms bound solidly with their neighboring atoms. They keep a set shape due to this tight "hugging" of atoms.
Liquids are made of atoms bound loosely with their neighboring atoms. The flow and settle to the shape of their container due to the loose "hand-holding" of atoms.
Gases are made of atoms not bound to other atoms. They flow and move freely due to their lack of bonds with their neighboring atoms.
Anything can be found in any one of these states of matter.
All you have to do to change the state of matter is change the energy. Cool water and it solidifies into ice; heat it and it vaporizes into steam.
Later this year we will explore liquid air!
But wait...
there's more...
What is BEC? What is plasma? Why have we not heard of them before?
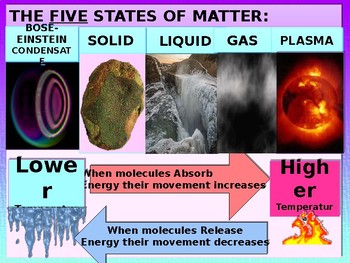
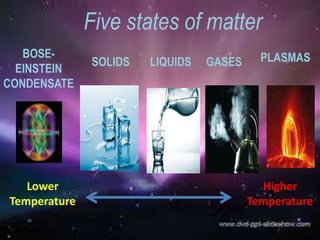
So plasma is when there is so much energy that the atoms fall apart into the charged particals that they are composed of. In our analogy that would be your limbs running around separately. The sun is made of plasma. Awesome, but not found much here on Earth.

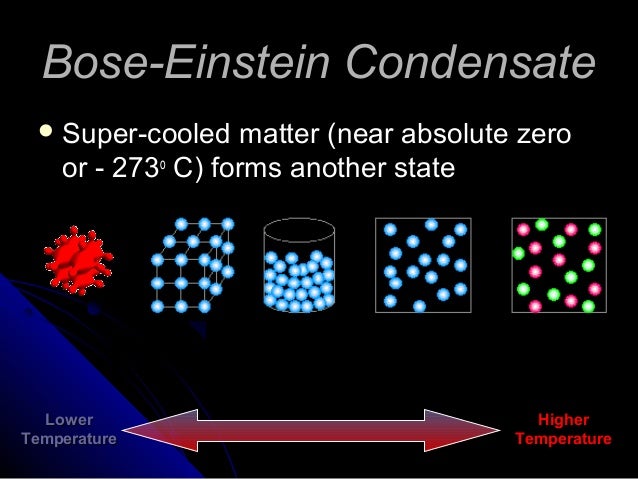
Bose-Einstein Supersolid Condensate is when the atoms are so cold and compressed that they behave as one atom instead of separate atoms (i.e. no resistence to electricity). This is not something you have ever seen or experienced here on Earth because it has to be soooo cold.
Are there other states of matter? Sure. Fermionic condensate, dark matter, etc. But, for our study, we will stick to these 5 states and how their atoms are interacting.
How do you convert a substance from one state to another? What is the name given for state changes?

Evaporation, condensation, melting, freezing, sublimation, and ionization are the common enough ones that you should be familiar with them.
Evaporation is a cooling process as the atoms with enough energy to turn into gas leave, taking their energy with them. That depletes the energy of what is left behind, leaving it cooler. That is why evaporation cools things (like us) off (that is why we sweat).
Condensation is a warming process for the same reason as evaporation is a cooling process. So, if you let condensation appear on the outside of your glass of water, it will warm the water inside up.
Air conditioning and refrigerators and some heating units use evaporation and condensation for their heating and cooling effects.
Matter has properties characteristic to them. A property is an attribute, quality, or characteristic of something.
Some of the properties of matter are density, hardness, temperature of state change, color, smell, burnable, reactivity, transparency, etc.
Some properties depend on the state of matter (i.e. something might be blue in liquid form but white in solid form and transparent in gas form)
Some states of matter have cool properties.
So lets begin with solids:
Solids connect in set structures:
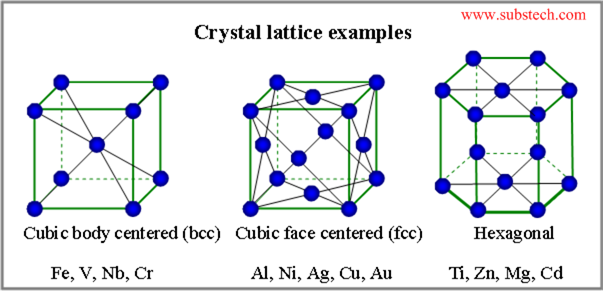
These structures are rigid, fixed, and not squishable.
We will play around with solids in class and create our own crystals.
The shape and cleavage (the way it breaks) can be properties of some solids as well.
Liquids have properties too:
Remember that liquids are only "holding hands" and that is loose, but how loose depends on the liquid and gives the liquid cool properties like:
Adhesion
Cohesion
Viscosity

Adhesion is how well a liquid sticks to other things. Think of adhesive tape--it sticks to other things.
Capillarity is a cool effect of adhesion (and what Einstein's first paper was on).
Cohesion is how well a liquid sticks to itself. Remind yourself that "co" means equal or same.
Surface tension is related to cohesion and provides some cool effects.

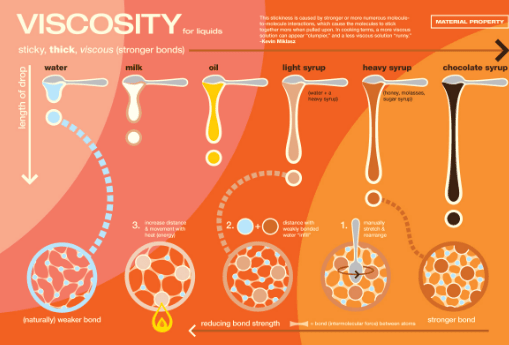
Viscosity it the liquid's resistance to flow (how thick it is).
Another property is dissolvability. This is how well it (solvent) dissolves other things (solutes). Think about putting sugar or salt in water and watching it dissappear.
Here is a more complete list (you do not need to know all of these):

Let’s play around with liquids
Gases have properties too.
Are all gases invisible? Do some have a smell? Does air have mass? How much space do gases take up?
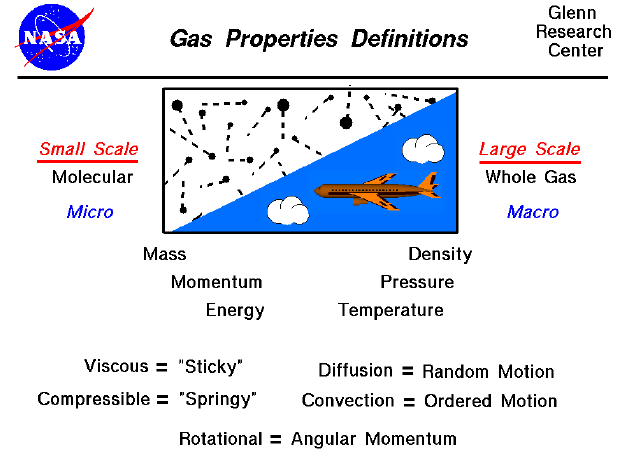
Gases also show viscosity.
The random movement causes diffusion (something moving from where there is a lot to where there is a little of it).
Gases are squishable.
Let’s play around with gases
Since both liquids and gases flow, they are called fluids and share some cool properties.
One was discovered by this man:

Archimedes!
As he climbed into the tub one day, water overflowed. But how much water came out? That is what was so cool. The weight of the water that came out equaled his weight. He was so excited by this and what it meant that he ran screaming down the street saying "Eureka!"

So, what was so exciting about this, he discovered that an object will float in a fluid when it displaces its weight of that fluid. Any object will float in any fluid when it moves away its weight of that fluid!
You float in water when you displace your weight of water. You would float in the air if you swelled to displace your weight of air.
This means that just about anything can float with the right shape. But what is the best shape. Let's experiment...
Do not look below until you have had a chance to explore...
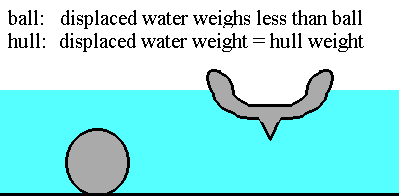
My father used to work on a ship made of heavy metal...yet it floated in water...how?
How can a hot air balloon lift you off the ground?
And how can a submarine float at times and sink at other times?
Another brilliant man discovered another property of fluids:

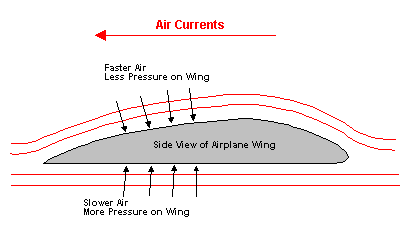
Daniel Bernoulli discovered something that has to be seen to be believed...

What is happening here!?
Bernoulli found that a moving fluid (air, water, etc.) exerts less force on an object. That means that the faster a fluid moves, the less that fluid pushes on you--you end up getting pulled towards the fast moving fluid.
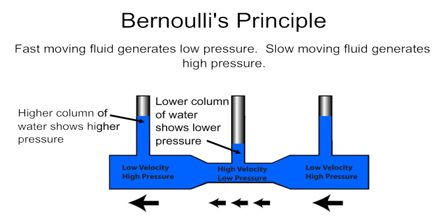
This allows for flight:
Why?
Bernoulli discovered that the faster a fluid is moving the less force it exerts.
You can think of it as blowing the fluid away from one side so only the fluid on the other sides push on the object --> therefore the object moves toward the faster moving fluid.
This allows flight and curve balls and other cool effects.


Both Bernoulli's and Archimedes's principles are based on that air has pressure.
Another property of fluids is Pressure. Pressure is the force pushing on an object equally in all directions.
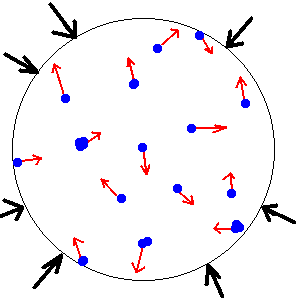
When immersed in a fluid (like the air we exist in), the fluid pushes in on an object from all directions. We call this pressure and it pushes equally in all directions. This means that the column of air above you right now pushes down on you with about the weight of a compact car. But it is also pushing the air next to you which causes that air to push on you sideways with the weight of a car (and so on and so forth in all conceivable directions--even up!). This upward pushing is called buoyancy and is what ultimately pushes planes and ships up in their respective fluids.

Mathematically, pressure is force / area
But we will wait until we learn about force for this one.
All matter has mass and takes up space so a property shared by all matter is density.
Mathmatically, density is Mass / Volume
Are you ready for the test?
Review questions:
Define matter.
List some properties of matter.
What are the states of matter?
What is happening at the molecular level in each state of matter?
What are the processes by which we convert one state to another?
Contrast adhesion and cohesion.
Define viscosity and fluid.
State Archimedes principle and how it allows for metal ships to float.
Define pressure.
State Bernoulli’s principle and how it allows for flight.
Calculate density.
If you have enjoyed the topics in this unit, feel free to investigate further. Here are some ideas. These are NOT required, but I hope you have fun and delve into some of them:
We touched upon the newest state of matter (Bose-Einstein super-solid condensate). What is it? What makes it different from a regular solid? Who discovered it? Research it.
Archimedes discovered and invented a lot in his day. Research him and some of his scientific breakthroughs. Maybe construct one of his inventions.
Which airplanes act like our paper airplanes (i.e. not using airfoils)? Why?
A refrigerator works by evaporation and condensation. Research it more to learn how and maybe build your own refrigerator (Cara did a few years ago).
Play around with buoyancy, adhesion, cohesion, etc. and look up a fun lab / activity the whole class can participate in.
Scuba divers deal with pressure a lot! What are “the bends”? How do divers protect themselves from the pressure?
Engineers design dams with pressures in mind. Look into how dams are built.
Flight obviously deals with pressure a lot. Research flight more. What is “wind shear”? How does a “flying wing” work?
Vehicle designers use aerodynamics to create faster and more efficient vehicles. Look into the design of some kind of vehicle.
Learn more about Bernoulli or Archimedes. They were fascinating people