Nuclear Energy
This unit will look at the energy released by nuclear reactions.
There are two types of nuclear reactions: Fusion and Fission
First, we should review the atom:
You will note that the Protons are all crammed in a tiny nucleus and have the same (repelling) charge. That means that they sometimes repel a proton out of the nucleus. This changes the atom and is called fission.


Note that two daughter atoms are produced. As are three neutrons. This breakdown happens at a set rate (the rates are different for different atoms though).
The energy comes in the form of Alpha particles, Beta particles, and Gamma rays:
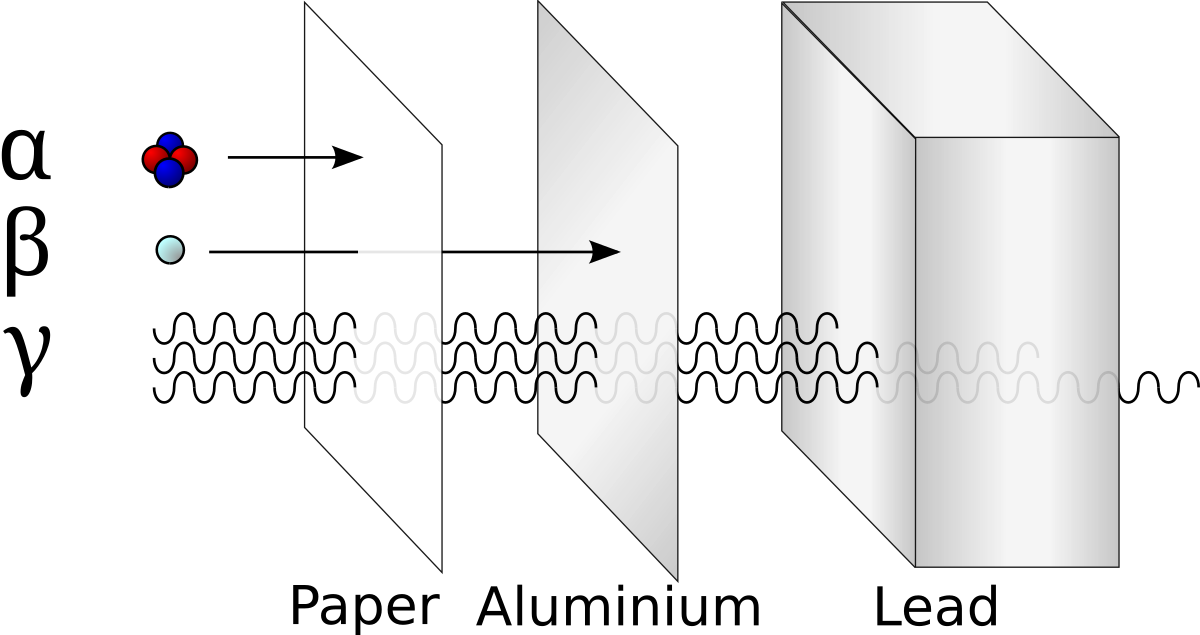
These products can be used in many, many ways! Here are some ways humans have used these products:
Bombs:
We discovered that hitting a fissile atom with a neutron makes it break down. And the breakdown releases three more neutrons. If we let each of these hit more atoms then we get a chain reaction.
Nuclear power:
This is the same as a bomb except with some rods that absorb some of the neutrons.
Medical tracers:

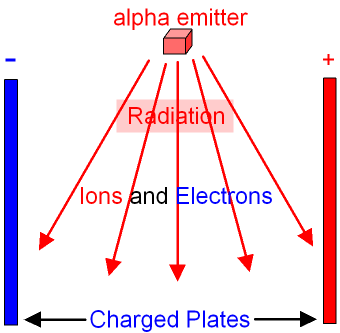
Or any leaks for that matter.
Smoke detectors:
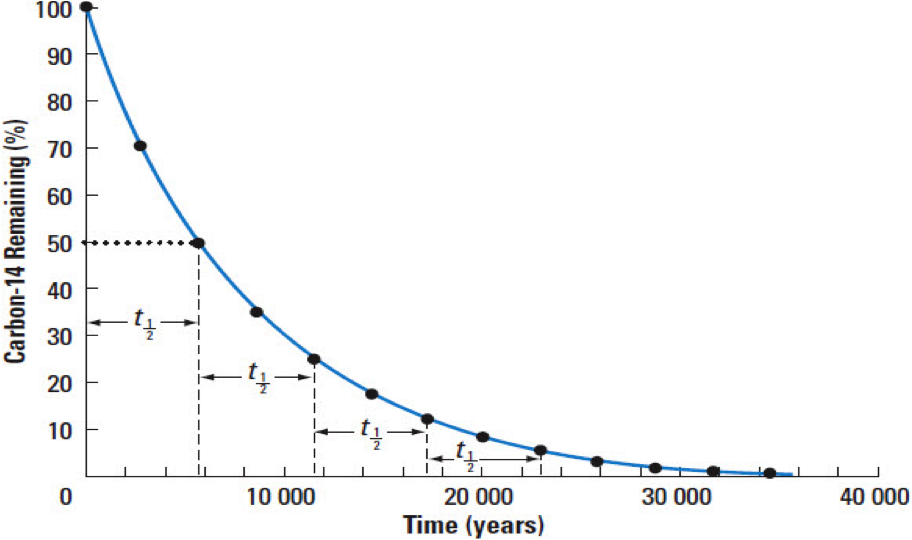
Because the breakdown is at a constant rate, we can date something by the ratio of parent atoms to daughter atoms:
Because radiation causes mutations and most mutations kill the mutated cell, if we blast cancer with radioactivity, we can kill the cancer.
So radiation is used to fight cancer.
Looking at the three neutrons that shoot out (chain reaction?), the alpha, beta, and gamma energy that is released, and the rate of parent / daughter atoms, what other uses can you find for radioactive decay?
Fusion:
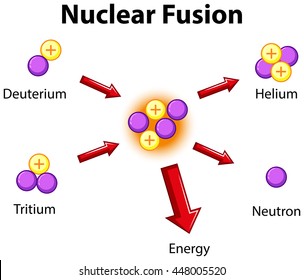
Did you notice what the fuel for fusion was?
hydrogen!
Did you notice the waste product for fusion?
helium!
Actually, you could use any fuel and produce any product. Burning helium to make gold, anyone?
So why are we not doing this?
What is stopping atoms from fusing?
The repulsion of the electrons and the tiny nature of the nucleus are the major hurdles in the way of fusion. If we heat something to plasma (ions with no electrons around and moving very quickly), or if we speed them up really, really fast, we can get them to fuse. Both require tremendous energy into by us. So much energy that it is not worth it. But if we could perform cold fusion...
This is the large Hadron Collider at CERN:
What are the two types of nuclear reactions
Contrast fusion and fission
What gets released when fission happens
What is a half life
What are some uses for radioactivity
